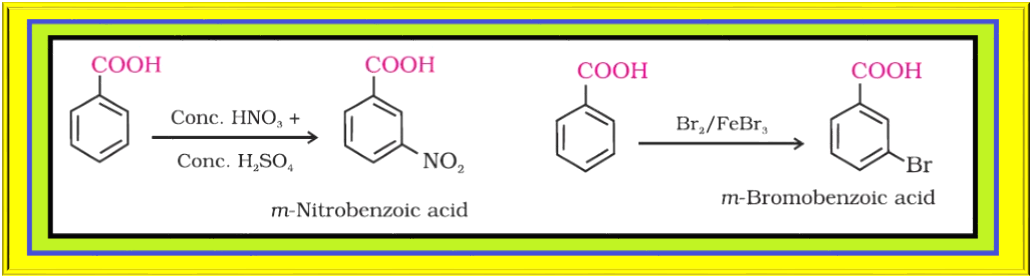
Decarboxylation :
`=>` Carboxylic acids lose carbon dioxide to form hydrocarbons when their sodium salts are heated with sodalime (`color{red}(NaOH)` and `color{red}(CaO)` in the ratio of `3 : 1`). The reaction is known as decarboxylation.
`color{red}(R- COONa undersettext(Heat) overset(NaOH & CaO)→ R- H +Na_2CO_3)`
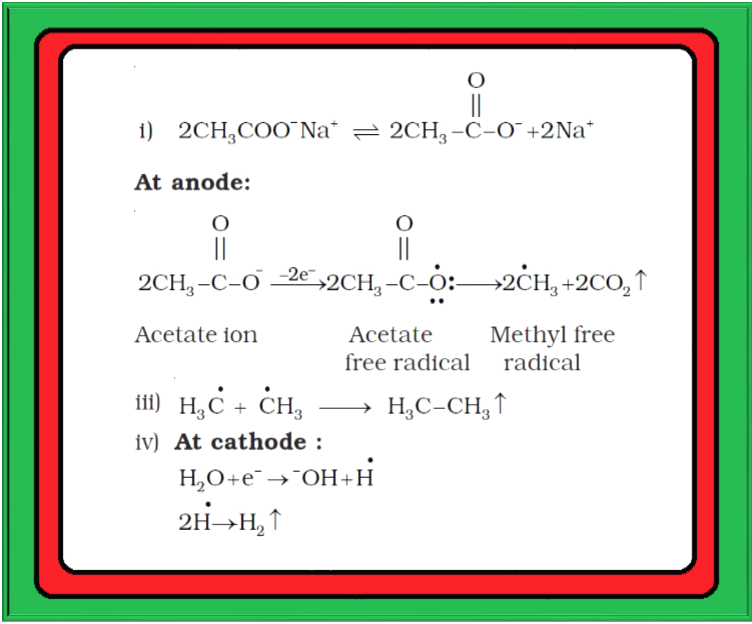
`=>` Alkali metal salts of carboxylic acids also undergo decarboxylation on electrolysis of their aqueous solutions and form hydrocarbons having twice the number of carbon atoms present in the alkyl group of the acid. The reaction is known as `color{green}(text(Kolbe electrolysis))`.
`color{red}(undersettext(Sodium acetate)(2CH_3COO^(-) text()^+Na) + 2H_2O oversettext(Electrolysis)-> CH_3 CH_3 + 2CO_2 + H_2 + 2NaOH)`
The reaction is supposed to follow the path shown in fig.
`color{red}(R- COONa undersettext(Heat) overset(NaOH & CaO)→ R- H +Na_2CO_3)`
`=>` Alkali metal salts of carboxylic acids also undergo decarboxylation on electrolysis of their aqueous solutions and form hydrocarbons having twice the number of carbon atoms present in the alkyl group of the acid. The reaction is known as `color{green}(text(Kolbe electrolysis))`.
`color{red}(undersettext(Sodium acetate)(2CH_3COO^(-) text()^+Na) + 2H_2O oversettext(Electrolysis)-> CH_3 CH_3 + 2CO_2 + H_2 + 2NaOH)`
The reaction is supposed to follow the path shown in fig.